기술동향
Drugging the Undruggable
- 등록일2011-04-06
- 조회수5702
- 분류기술동향 > 종합 > 종합
-
자료발간일
2011-04-04
-
출처
Technology Review
- 원문링크
Drugging the Undruggable
A chemical "staple" creates mobile biological molecules with staying power.
While scientists have identified proteins that drive myriad diseases, finding ways to control them is another matter. Thanks to structural and chemical limitations, only 20 percent of the body's proteins can be targeted with existing drugs. Most existing pharmaceuticals are either small molecules that require very specific surface features to enter a cell or large biological molecules that are too big to gain entry. But new types of therapeutic molecules developed over the last decade, called stapled peptides, may be able to work their way into tissues that were previously inaccessible. In research presented at the American Chemical Society meeting in California last week, Harvard biochemist Gregory Verdine described two potential new drugs—one for colon cancer and one for asthma—that are capable of going where none have gone before.
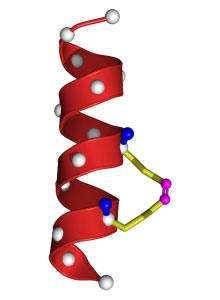
Stapled Up: A chemical bond (yellow) between two successive turns of this peptide (red) acts as a "staple," creating a stable molecule that can enter cells that are inaccessible with current therapeutics.
Credit: Verdine Laboratory, Harvard University
"The world of biology has rocketed ahead to explain the proteins that cause disease, but the world of targeting and chemistry has not kept pace," Verdine says. "What we need are new types of molecules that are able to gain access to the inside of cells and that have the kind of surface properties that allow them to target things that small molecules can't."
The most successful of today's therapies work by interacting with receptors on a cell's surface, or by using small molecules that can enter a cell via water-phobic "greasy" pockets. The problem, however, occurs when cells lack the necessary surface features. For example, proteins responsible for tumor cell growth have been difficult to inhibit via traditional means. To solve the problem, Verdine turned to short pieces of protein called peptides, which can switch disease pathways within the cell on or off and can also be manipulated into shapes that slip easily into a cell's nucleus.
The idea of manipulating peptides into therapeutics is not new, but the problem has been that they're notoriously unstable. They tend to lose their shape once isolated from their parent protein and are rapidly broken down by the body's enzymes. Verdine and his collaborators have gotten around this problem by folding a peptide into shape and holding it there by strategically placing two amino acids on the peptide and linking them together into a chemical "staple." The result is a strong, stable, helical peptide that can penetrate a cell's interior and retain its shape long enough to have therapeutic effects.

