기술동향
Unpicking HIV’s invisibility cloak
- 등록일2012-02-14
- 조회수7059
- 분류기술동향
-
자료발간일
2012-02-10
-
출처
Riken Research
- 원문링크
-
키워드
#HIV#invisibility cloak#Unpicking #cloak#therapeutic
Unpicking HIV’s invisibility cloak
Revelation of how certain compounds adhere so strongly to HIV’s coat points to a fresh therapeutic approach
Drug researchers hunting for alternative ways to treat human immunodeficiency virus (HIV) infections may soon have a novel target-its camouflage coat. HIV hides inside a cloak unusually rich in a sugar called mannose, which it uses to slip past the immune system before infecting its host’s cells. Recently, however, biochemists discovered a family of chemical compounds that stick strongly to mannose. Understanding how this mechanism works could reveal a way to make drugs adhere to and kill HIV. Yu Nakagawa and Yukishige Ito at the RIKEN Advanced Science Institute in Wako and their colleagues from several research institutes in Japan are leading the effort: they have mapped the binding site of the mannose-binding compound pradimicin A1.
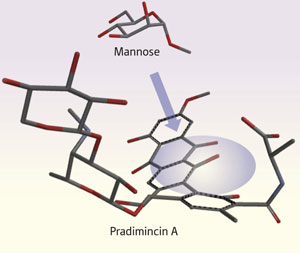
Figure 1: The compound pradimicin A disrupts the human immunodeficiency virus (HIV) by clinging to its mannose-rich coat. The mannose sits within a cavity in the pradimicin A structure (purple shading).
Mannose-binding compounds are particularly attractive to drug researchers thanks to their double-action anti-HIV effect. By sticking to mannose in the virus’s coat, pradimicin A first freezes HIV’s molecular machinery for entering and infecting its host’s healthy cells. The virus responds by reducing the mannose in its coat thereby revealing itself to the immune system, which can then attack.
Unraveling just how pradimicin A recognizes mannose, however, has proven surprisingly difficult. In solution, the two components stick together in variously sized small clusters, confounding conventional analytical techniques such as solution-based nuclear magnetic resonance (NMR) and x-ray crystallography. Nakagawa, Ito and their colleagues side-stepped the clumping problem by using solid-state NMR, which allowed them analyze the compounds as solids, rather than in solution.
The research team’s approach involved inserting carbon-13, a chemical label, into particular parts of the pradimicin A structure. Carbon-13 boosts the NMR signal wherever it is inserted, so the team could ‘walk’ around the compound and detect where it interacts most strongly with mannose.
The results revealed that pradimicin A curls up to form a cavity, within which the mannose structure sits (Fig. 1). “Our study highlights the benefit of solid-state NMR methodology to investigate this interaction,” says Nakagawa. “Solid-state NMR is, at present, the only technique to analyze such a complicated system.” Flagging the potential utility of the technique, Nakagawa adds that: “Our analytical strategy might be applicable to other systems that similarly suffer from aggregation in solution.”
Meanwhile, solid-state NMR can offer even more in probing mannose?pradimicin A binding, Nakagawa says. Having determined how and where pradimicin A grabs mannose, the team’s next step will be to use the technique to identify the specific molecular interactions that bind the pradmicin A to this potential Achilles' heel of HIV.
The corresponding author for this highlight is based at the Synthetic Cellular Chemistry Laboratory, RIKEN Advanced Science Institute
지식
- BioINwatch HIV를 제어하는 슈퍼 면역인 '엘리트 컨트롤러' 2020-10-19
- BioINdustry 암 항체 미국 특허 획득,만성 폐쇄성 폐질환 약 유럽 승인 획득 등.. 2010-07-27
- BioINdustry HIV 치료제 FDA 전통적 승인 획득, 결막염 치료제 유럽 판매승인 획득 등.. 2009-12-15
- BioINdustry 신경질환 후보물질 미국 특허 출원 허가서 획득,안면홍조 임상 3상 긍정적 결과 보고 등.. 2009-10-19
- BioINdustry 아프리카의 유전자변형 작물 논쟁, HIV-1의 새로운 구조기 발견 등.. 2009-09-16

