Korean Biotechnology Industry:
Its Past and Future Outlook
Private Sector Collaboration
Private sector: During this period, Korean companies have utilized the results of basic research in bioscience and biotechnology to strengthen cooperation among innovation actors. This has led to innovative outcomes and new business opportunities. By pursuing global strategies, Korean companies have successfully established an innovation system and infrastructure that supports sustainable growth.
Goverment: Over the past forty years, the Korean government has consistently adjusted and improved national policies and directions in biotechnology to actively respond to paradigm shifts driven by technological development and innovation.
Korean Biotechnology Now Preparing for a Giant Leap onto the Global Stage
Securing technological and industrial capabilities to lead the Bio-Transformation
In the IMD (International Institute for Management Development) World Competitiveness Ranking of 2023, Korea was ranked 2nd in scientific infrastructure competitiveness (3rd in the previous year) and 23rd in technological infrastructure competitiveness (19th in the previous year). As of 2022, Korea’s technological level in healthcare and industry-related technologies stands at 79.4% of that of the U.S., the world's leading country, with a technological gap of 2.5 years. Compared to 2016, Korea has shown a 3.3% improvement in its technological level, which was at 76.1% back then. Furthermore, the technological gap between Korea and the U.S. has shortened by 0.7 years from 3.2 years in 2016.
Source: The World Competitiveness Yearbook, IMD, 2023; 2022 Expert Survey on
the
Assessment of Healthcare and Industry-related
Technologies and Its Results Analysis, Korea Health Industry Development Institute (KHIDI), November
2022

Securing growth momentum through active global technology transactions and M&As
-
Technology Exports in Bio Sector (2017-2021)

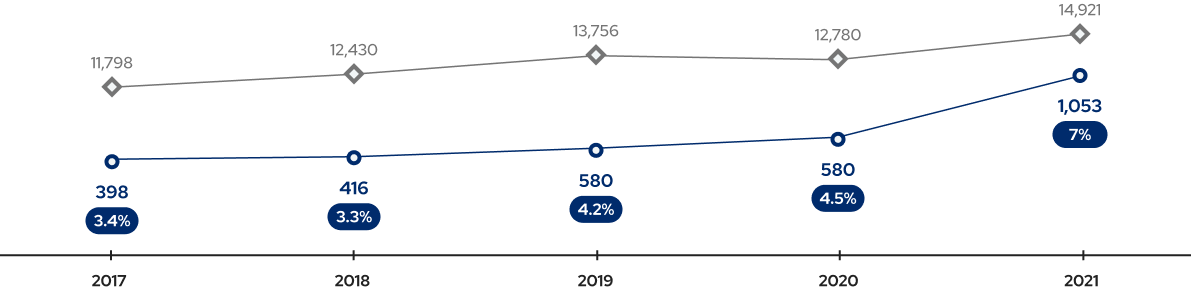
Source: Technology Trade Statistics, Ministry of Science and ICT, 2017-2021
Explosive growth of biopharmaceuticals driven by rapidly increasing sales and production demand
-
Biosimilar Sales

Source: Samsung Biologics; Celltrion
-
Global Market Share of Domestic Conglomerates in the
Biotechnology
Industry

Source: 2030 Innovation Strategy Leeading the Bio Transformation under the 4th Framework Plan for Biotechnology Promotion jointly developed by releevant ministries, 2023
Korea’s bioindustry and healthcare sector: Opening new horizons in digital innovation
Korean Biotechnology Sector-Key
Competencies for Future Growth
Current Competitiveness of the Korean Biotechnology Sector
-
Government and Private Sector R&D Investment in Biotechnology
(2017-2021)
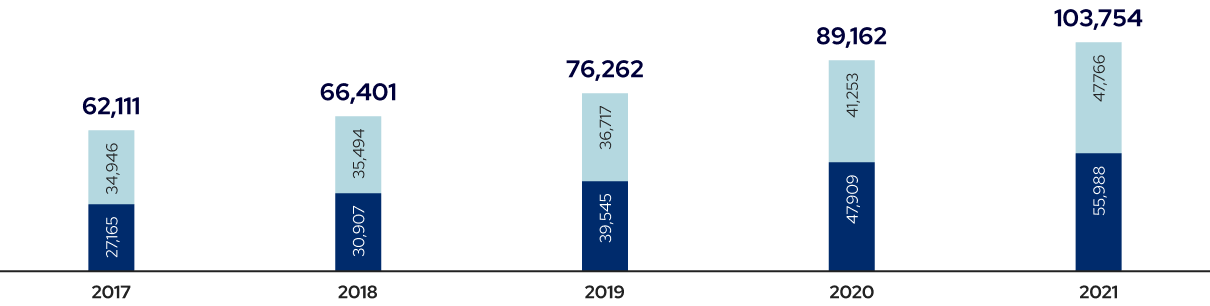
Source: Survey Report on R&D Activities, 2017-2021, KISTEP; Reprocessed by Biotech Policy Research Center, KRIBB
-
Number of Personnel Employed in the Biotechnology Industry by Year
(2020-2022)
Classification 2020 2021 2022 CAGR No. of personnel 52,297 56,718 61,152 8.1 Growth rate 7.4 8.5 7.8 Source: 2022 Survey of Domestric Bioindustry, 2023, jointly published by the Ministry of Trade, Industry, and Energy (MOTIE) and KoreaBio
-
Korean SCI Publications by Year (2017-2021)
Classification 2017 2018 2019 2020 2021 No. of SCI publications 61,671 64,481 70,738 76,874 84,070 Percentage in the global total (%) 3.37 3.40 3.34 3.39 3.47 No. of SCI publications 12th 12th 12th 12th 12th Source: InCities DB; WoS(Web of Science); ESI (Essential Science Indicators, 2011-2021); Reprocessed by the author
-
Biotechnology Patents Registered in the U.S. by Country of Inventor
(2013-2022)
2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 Total CAGR U.S. 6,267 6,663 6,482 6,470 5,924 6,781 7,329 7,309 6,935 6,157 66,317 0.20% Korea 218 248 255 266 301 354 377 388 324 370 3,101 6.05% Source: Korean Intellectual Property Office (KIPO)
Biotechnology companies among the KOSDAQ-Listed Technology Special Companies
Since 2005, the KOSDAQ market has implemented a special preliminary review exception program for technology companies, specifically designed to allow those with minimal sales performance but significant technological prowess and growth potential to be listed. This program enables companies to be listed through a technology evaluation by specialized evaluation agencies or recommendations by listing agents.
As of 2022, a total of 171 technology-specialized companies have been listed under this program, 101 of which are biotechnology companies.
Building a World-Class Ecosystem for Biotechnology Industry
- No. of companies
- Human resource
- Investment
- Sales
Units: No. of companies, Headcount, KRW tril.
Source: 2022 Biotechnology Industry Survey by the Ministry of Trade, Industry and Energy (MOTIE) and the KoreaBiotechnology Industry Organization, 2023
Scope of the survey: Korean companies engaged in business activities related to biotechnology and bioengineering according to the Biotechnology Industry Classification Code, KS J 1009. This code was endorsed by the Korean Agency for Technology and Standards under the Ministry of Trade, Industry and Energy in January 2008 and revised on December 29, 2016. The classification code provides standards for defining the scope of biotechnology.
Companies surveyed: Out of 1,089 registered companies in the Korean biotechnology industry, 1,074 biotechnology companies participated in the survey, while 15 did not respond.
Human resource: Refers to the number of employees hired in the Korean biotechnology industry.
Investment: Refers to the total investment in the Korean biotechnology industry, including R&D and facility investment.
Sales : Includes both domestic sales and exports of biotechnology companies.
Government’s Efforts to
Foster Biotechnology
Government Strategies for Biotechnology Innovation and Globalization
Framework Plans and comprehensive Plans
The 4th Framework Plan for Biotechnology Promotion : Innovation Strategy 2030 Leading the Bio-Transformation
The 4th Framework Plan for Biotechnology Promotion (2023-2032) has been collaboratively developed by 14 ministries, including the Ministry of Science and ICT, in accordance with the Biotechnology Promotion Act. This plan is the highest statutory framework governing the nation’s biotechnology R&D.
Through the bio-transformation often characterized by 'convergence' and 'connection', Korea is able to overcome current limitations and create new opportunities that span across all areas of the bioindustry.
The 4th Framework Plan for Biotechnology Promotion is a comprehensive policy aimed at supporting all areas of biotechnology throughout the entire technology development stage, with the ultimate goal of positioning Korea as a leading nation in the global bio-economy.
-
Basic Direction of the Framework Plan
Source: Ministry of Science and ICT
-
The 4th Framework Plan for Biotechnology Promotion: Vision,
Goals, and Initiatives
Source: Ministry of Science and ICT
The 3rd Master Plan for Fostering Health and Medical Service Technology
- The 3rd Five-Year Comprehensive Plan for the Development and Support for Bio-Pharmaceutical Industry as a Bio Health Global Powerhouse
- Announcement of New Bio-Health Industry Regulation Innovation Plan
- Healthcare 4.0 Era for Healthy Korea
1st Comprehensive Plan for Development & Support of the Medical Devices Industry
Strategies to respond to the digital transformation of bioindustry
Joining the ranks of leading countries by 2030 using digital biotechnology in the era of bio-transformation : Digital Biotechnology Innovation Strategy (December 2022)
The Yoon administration set a goal of "joining the ranks of G5 countries in science and technology by fostering super-gap strategic technologies" as part of its 110 National Agendas, which includes "promoting digital biotechnology for bio-transformation".
The strategy has been developed to help Korea achieve this goal by overcoming the limitations of existing biotechnology R&D programs, such as high costs, high risks, and long lead times. The strategy aims to promote Korea’s entry into the ranks of biotechnology leaders through the tactics of selection and focus.
-
Digital Biotechnology Innovation Strategy: Vision and
Strategies
Source: Ministry of Science and ICT
Strengthening biomanufacturing innovation capabilities in the era of engineering biology: Engineering biology is a key solution for overcoming the limitations of bioresearch (November 2022)
- Korea to announce the National Synthetic Biology Initiative
- Biofoundry Infrastructure Establishment Project Passes Preliminary Feasibility Study