기술동향
Stacking at the flick of a switch
- 등록일2009-12-14
- 조회수6805
- 분류기술동향 > 종합 > 종합
-
자료발간일
2009-12-14
-
출처
RIKEN Research
- 원문링크
-
키워드
#유전자발현#gene expression#molecular machines
Stacking at the flick of a switch
A switch that controls formation of stacks from nucleic acid strands has potential applications in gene expression and molecular machines
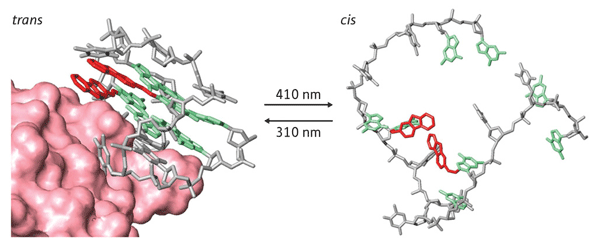
Figure 1: Schematic of the reversible switch. The aptamer on the left, which has a so-called G-quadruplex structure, is bound to thrombin (pink). Light-induced photoswitching leads to destabilization of structure and loss of binding; the process can be reversed via further irradiation.
Small, structured nucleic acid strands designed to interact with specific target molecules, known as aptamers, are particularly interesting to scientists as therapeutics against viruses and blood clotting disorders. Aptamers that bind strongly to target enzymes or proteins can prevent their activity, thereby regulating the biological process they control.
Aptamers frequently contain stacks of hydrogen-bonded guanine-rich sequences, called quadruplexes, which enable effective binding to targets. When these structures are disrupted or destroyed-perhaps through poor replication-the aptamer can no longer bind.
Interestingly, the reversible formation and disruption of these quadruplexes have the potential to be controlled using external stimuli, leading to a switching on and off of the aptamer's binding. Using this idea, Shinzi Ogasawara and Mizuo Maeda at the RIKEN Advanced Science Institute in Wako, have developed a light-controlled switch to control quadruplex formation in an aptamer that binds specifically to thrombin1, the protein in blood that control blood coagulation.
The researchers developed a range of modified aptamers that contained various numbers of potential quadruplex structural units. Using light they were able to switch and between a stable quadruplex and a non-structured state. Using light to control a switch allows accurate and easy control of the location, dosage, and timing. They then screened their variant aptamers to determine the best number and positions of the structural units to give effective regulation.
More specifically, Ogasawara and Maeda were able to control the switching behavior using light of specific wavelengths. In solution, they found that when the quadruplex is present, the aptamer binds strongly to thrombin. When the sample was irradiated at 410 nanometers, the double bonds within the complex d from a so-called trans configuration to a cis configuration that disrupted the quadruplex and suppressed binding of the aptamer (Fig. 1). Irradiation at 310 nanometers, however, d the bonds back into the trans form, allowing the quadruplex to re-form and bind once again.
Ogasawara and Maeda realized that the release-and-bind steps could be repeated over two cycles. First, they irradiated a reaction sample at 410 nanometers for 5 minutes and then at 310 nanometers for 2 minutes. After repeating the irradiation cycle, they found that the switch was completely reversible, and they detected no side reactions, thus demonstrating its potential in living cells.
Ogasawara and Maeda now plan to apply this technique to investigate other important biological events involving quadruplexes, such as gene expression and the construction of molecular machines.
The corresponding author for this highlight is based at the Bioengineering Laboratory, RIKEN Advanced Science Institute
1. Ogasawara, S. & Maeda, M. Reversible photoswitching of a G-quadruplex. Angewandte Chemie International Edition 48, 6671?6674 (2009). article
지식

