기술동향
[김영수] RNA-targeted Therapeutics in Oncology
- 등록일2019-06-03
- 조회수5887
- 분류기술동향
RNA-targeted Therapeutics in Oncology
(Ionis Pharmaceuticals, 김영수)
[목차]
1. Introduction
2. RNA-targeted Therapeutics: ASOs vs siRNAs
3. Advances in medicinal chemistry
4. Clinical experience of RNA-targeted therapeutics in oncology
5. Challenges and opportunities of RNA-targeted therapeutics in oncology
6. Conclusions
7. References
[내용]
1. Introduction
Based on Watson-Crick rule, the feasibility of modulating gene expression by hybridizing target RNA sequences with oligonucleotides was initially demonstrated in Rous sarcoma virus1. Since then, the concept of selectively modulating the expression of disease-causing genes at RNA level has evolved to the development of a novel therapeutic approach. Especially, after the completion of whole human genome sequencing which showed >98% of genes are not translated2, the list of potential targets amenable to this technology has been expanded tremendously, from simple protein coding genes to noncoding genes such as miRNAs and long noncoding RNAs (lncRNAs).
Because of no limitation in target space and the specificity this technology can provide, drug companies have tried using oligonucleotides to treat a variety of pathological conditions ranging from infectious diseases, rare genetic diseases to cancer. However, it took nearly 4 decades until the technology finally established itself as a valid drug platform after failing in multiple late-stage clinical trials because of either insufficient efficacy or sporadic toxicities.
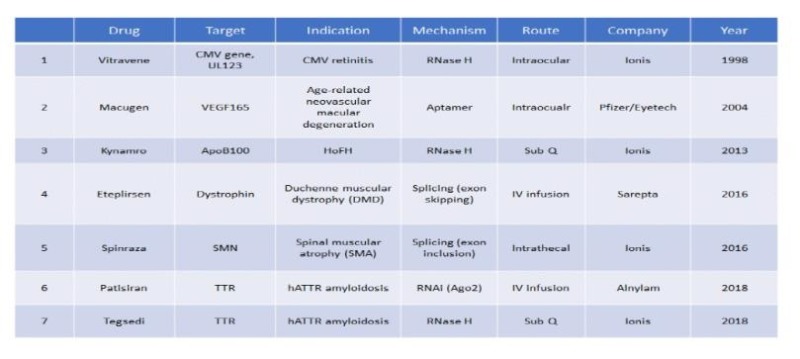
There are currently seven U.S FDA-approved RNA-targeted therapeutics (Table 1), four of which have been approved within last three years. Due to impressive clinical and commercial success of one of the drugs, Spinraza® for the treatment of spinal muscular atrophy (SMA)3, a rare childhood neurogenerative disease, the enthusiasm for this drug platform has never been higher than now. Since most readers may not be familiar with this drug platform and major clinical advances leading to FDA-approvals have been made for non-oncology indications thus far, this review will first summarize the evolution of the technology, the mechanisms of action of drug, as well as discuss challenges and future opportunities it has, with a focus on oncology.
2. RNA-targeted Therapeutics: ASOs vs siRNAs
RNA-targeted therapeutics can be largely classified into two types of drugs; antisense oligonucleotides (ASOs) and small interfering ribonucleic acids (siRNAs). Principally, both types of drugs induce gene silencing through anti-sense mechanism. However, they differ from each other in many aspects, which has become major determining factors in their respective clinical applications4, 5.
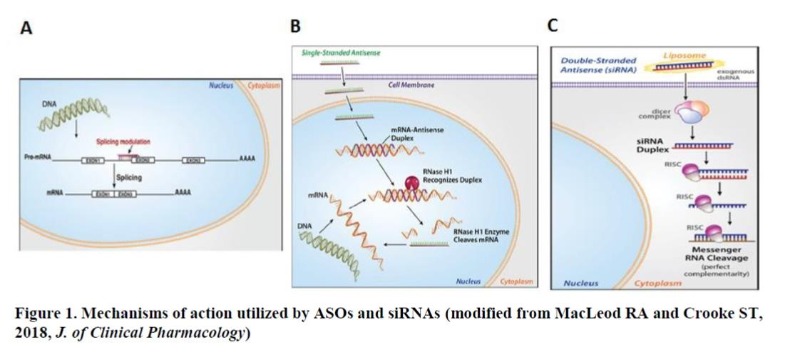
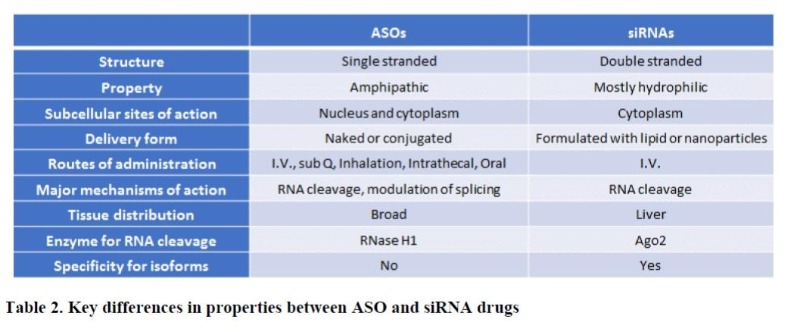
As a single strand, ASOs exert their effects on gene expression through two major mechanisms. One is based on simple occupancy of the cognate target RNA sequence following binding (RNase H1-independent), through which several processes including RNA splicing, translation, and interaction of RNA with proteins can be modulated6 (Figure 1A). ASOs utilizing this mechanism is typically composed of fully modified nucleotides to increase stability. Eteplirsen® for Duchenne muscular dystrophy (DMD) and Spinraza® for SMA are two examples of such ASO drugs which treat the respective disease by altering the splicing of target genes, dystrophin and survival motor neuron (SMN), respectively. The other mechanism relies on the occupancy-mediated degradation of the target RNA by ASO (RNase H1-dependent), where the heteroduplex formed by ASO and its target RNA is recognized by an enzyme RNase H1, leading to the cleavage of RNA5 (Figure 1B). In fact, this is the most widely used mechanism by ASO drugs currently in clinical development for various indications including cancer. Kynamro® for the treatment of homozygous familial hypercholesterolemia (HoFH) and Tegsedi® for the treatment of hereditary ATTR (hATTR) amyloidosis are two of FDA-approved drugs that depend on RNase H1 for their actions. In contrast to ASOs, siRNAs are composed of double strands with a sense or “passenger” strand and an antisense or “guide” RNA. Inside the cells, the sense strand is degraded and the antisense strand is loaded into a gene-silencing protein complex called “RISC”, where the guide strand binds to the cognate target sequence and the target RNA is subsequently cleaved by an endonuclease, Ago27 (Figure 1C). Since the RNA cleavage mediated by Ago2 occurs mostly in the cytoplasm, siRNAs are ineffective in modulating splicing events or downregulating the expression of nuclear-retained transcripts such as long noncoding RNAs (lncRNA). Patisiran® became the first FDA-approved siRNA drug in 2018 for the treatment of hATTR amyloidosis, the same indication approved for Tegsedi. While ASOs are active and broadly distributed to different tissues without any formulation in both cell culture and following systemic administration, siRNAs need to be formulated for efficient delivery to the cells due to their hydrophilicity8. Moreover, siRNA drugs are mostly accumulated in the liver. This lack of intrinsic activity of siRNA without formulation has become a big hurdle to overcome for its application in targeting extrahepatic tissues, especially tumors. Some of key differences between the two drug entities are listed in Table 2.
3. Advances in medicinal chemistry
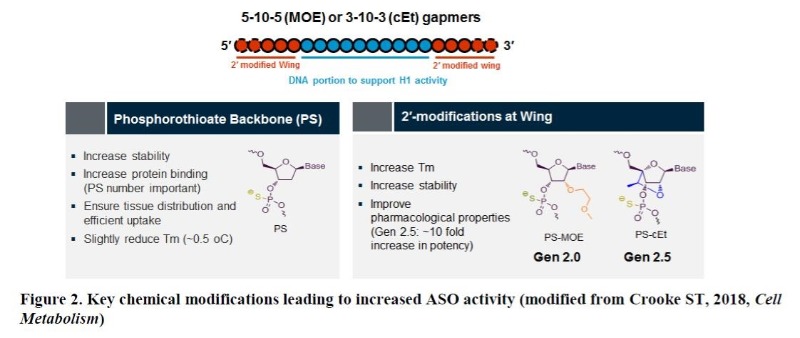
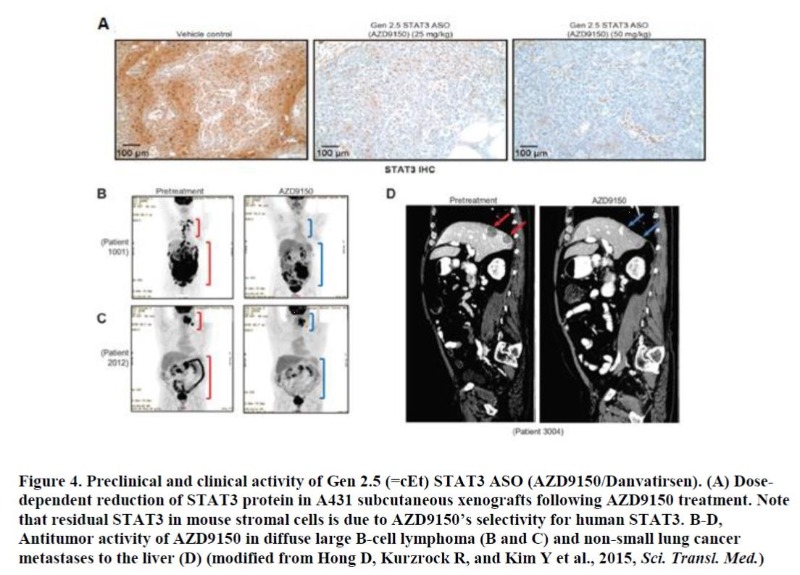
Natural oligonucleotides without appropriate chemical modifications are rapidly cleaved by both cellular and extracellular endo-/exonucleases5. Therefore, it is conceivable that medicinal chemistry has played a key role in enhancing the drug properties of ASOs through improvements in both potency and safety (Figure 2). Two sites of ASOs have been the focus of such chemical modifications; the linkage and the sugar. The substitution of phosphodiester (P=O backbone) with phosphorothioate (P=S backbone) in the linkage led to increased stability and to reduced hydrophilicity of ASOs5. Thus, ASOs with such modification can bind to proteins more efficiently in the serum and also intracellularly, resulting in broad distribution of the drug in the body following any routes of delivery9. Although numerous modifications on the sugar have been evaluated, 2’-methoxyethyl (2’-MOE or Gen 2 chemistry) substitution at each end (wing) of ASO has turned out to be most efficient in enhancing ASO activity with favorable safety profiles5. Recently, constrained ethyl (cEt or Gen 2.5 chemistry) modification on the sugar instead of 2’-MOE demonstrated significant increases in STAT3 ASO activity (5 to 10-fold) largely due to its higher affinity for target RNA, especially in rather less amenable tissues to ASOs such as tumors10. Since a stretch of nucleotides (typically 10= also called ‘gap’) in the middle is unmodified to support RNase H1-mediated cleavage, this type of ASO is called a MOE or cEt “gapmer” depending on the type of sugar modification.
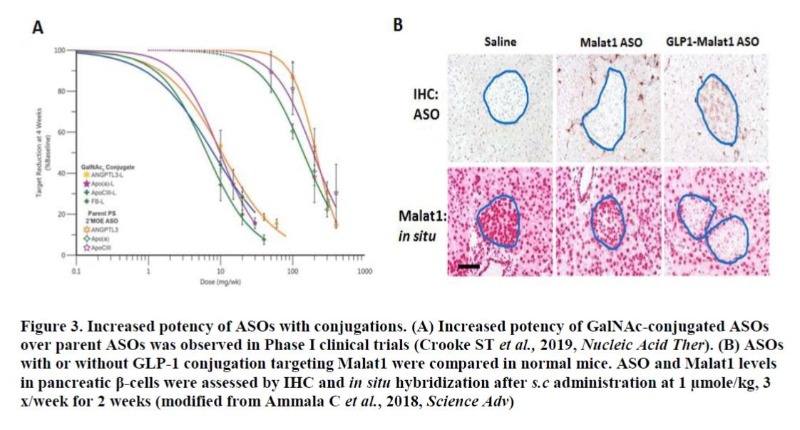
Selectively delivering a drug to the cell type of interest can improve drug’s property tremendously by increasing drug levels in target tissue(s) while minimizing the accumulation of drug in unwanted tissues, thus mitigating any potential on-target toxicity. Some clinical successes have been achieved by conjugating a drug with an antibody or a ligand for a receptor expressed highly in certain types of tumors or tissues11. Recently, the feasibility of applying such targeted-delivery approach to ASO has been demonstrated. Asialoglycoprotein receptor (ASGR) is a cell surface protein abundantly expressed in hepatocytes. Conjugation of ASOs with N-acetyl galactosamine (GalNAc), a ligand for ASGR, led to strong improvement in ASO activity in liver for multiple hepatic targets in both preclinic animal models and humans12, 13 (Figure 3A). Similar improvement was also demonstrated with siRNA drugs conjugated with GalNAc targeting hepatic genes14. Typically, ASO activity in pancreatic beta-cell is very limited following systemic delivery. Conjugation with glucacon-like peptide-1 (GLP1), a ligand for GLP1-receptor (GLP1-R) led to robust ASO activity in beta cells with an evidence of significant drug accumulation15 (Figure 3B). These so-called “LICA” (ligand-conjugated ASO) approaches are certainly game-changing findings in ASO technology and efforts to identify such ligand-receptor systems highly sensitive to ASO are expected to further intensify. While the main focus of medicinal chemistry in ASOs has been on increasing the intrinsic potency/safety of drug, siRNAs have had to make enormous efforts to improve the intrinsically poor pharmacokinetic properties the drug class possesses. Through various formulations with lipid and/or nanoparticle, the activity of siRNAs has been markedly improved at least in hepatic tissue 16.
4. Clinical experience of RNA-targeted therapeutics in oncology
Despite remarkable evolution in cancer therapies from nonselective cytotoxic drugs to targeted- and recent immune-oncology drugs, many of “drivers” for tumor growth such as a variety of transcription factors are still undruggable by conventional therapeutic modalities. Furthermore, increasingly available genomic data from each cancer patient also points the importance of applying “personalized medicine” for maximal efficacy. In these regards, RNA-targeted therapeutics are poised to be an effective drug platform for treating tumors unamenable to currently available therapies based on patients’ genomic data. Prior to the introduction of Gen 2.5 chemistry, however ASO drugs with earlier chemistry (Gen 1 and Gen 2) or with a different sugar modification (e.g. locked nucleic acid=LNA) suffered from failures in multiple clinical trials due to either lack of clinical activity or hepato/renal toxicity17, 18. One thing we should keep in mind is that those ASOs with earlier chemistry are far inferior to highly optimized Gen 2.5 ASOs by today’s standards for potency and safety. Over the course of developing ASO drugs, the capacity for predicting and screening out potential toxic compounds at preclinical stage has been greatly increased. Therefore, readers should be careful not to view such failures as a common limitation among all types of ASO drugs in clinical trials with different chemistries. Compared to ASO drugs, the experience siRNA drugs have accumulated in oncology is still at an infancy with certainly higher hurdles to overcome, especially in delivery to tumor. In fact, some of toxicity observed in siRNAs during clinical trials could be attributed to the formulations the drugs had to adopt for efficient delivery19. Highlights of recent clinical results from RNA-targeted therapeutics in oncology are described below.
– STAT3 ASO (AZD9150/Danvatirsen
STAT3, a transcription factor of STAT family, regulates the expression of multiple genes implicated in tumor biology. Even though STAT3’s role in promoting tumor growth mediated by tumor cells was mainly appreciated in the past, recently its contribution to tumorigenesis via tumor-associated stromal cells especially through immune cells has become an area of active research20. There is a growing body of evidence that activation of STAT3 in various types of immune cells can create immune-suppressive tumor microenvironment, dampening antitumor effects from chemo-, targeted-, and especially immune-oncology therapies21. Despite its essential role in tumorigenesis, developing a STAT3-specific inhibitor has not been tractable. AZD9150 (Danvatirsen) is the 1st ASO entering the clinical stage with cEt (Gen 2.5) chemistry (developed initially by Ionis Pharmaceuticals Inc. and later licensed to AstraZeneca). As described earlier, AZD9150 demonstrated a significant improvement in potency in multiple preclinical tumor models compared to a STAT3 ASO with 2’-MOE chemistry (Figure 4A). When evaluated in phase I clinical trials, AZD9150 demonstrated significant efficacy in several types of treatment refractory tumors as a single agent, most notably in advanced lymphoma patients with several partial and complete responses22 (Figure 4 B-D). Based on STAT3’s role in immune suppression which was further supported by preclinical studies performed in syngeneic mouse tumor models using a mouse STAT3-specific ASO, AZD9150 is currently being evaluated in combination with PD-L1 inhibitor, Durvalumab in multiple phase 2 clinical trials for the treatment of advanced tumors, with an encouraging early sign of efficacy23. More Gen 2.5 ASO drugs targeting key survival genes in different cancer types (e.g. androgen receptor for advanced prostate cancer) are either currently being evaluated in or about to enter clinical trials.
– siRNA drugs
Even though numerous studies demonstrating pharmacological activity of siRNAs in preclinical models of cancer have been published for last two decades or so, very few drugs managed to reach clinical stage, and most of which failed in early clinical trials largely due to insufficient activity. For example, TKM-080301 is a siRNA drug encapsulated with lipid nanoparticles targeting polo-like kinase I. It showed encouraging antitumor activity in a phase I study in patients with advanced hepatocellular carcinoma (with 22% partial response), however it failed to demonstrate any meaningful clinical activity in a dose escalation study with Phase II expansion cohort24. siG12D-LODER is a siRNA drug selective for mutant form of K-RAS. It is encapsulated in a polymer Local Drug Eluter (LODER) for prolonged delivery to tumors. Evidence of drug’s distribution to PDAC following intratumoral injection was demonstrated in a phase I clinical trial25. The drug is currently being evaluated in patients with unresectable locally advanced pancreatic cancer in combination with gemcitabine in a phase II trial.
5. Challenges and opportunities of RNA-targeted therapeutics in oncology
Despite enormous potential the technology has in treating tumors with higher selectivity and safety than conventional drug modalities, clinical successes with RNA-targeted therapeutics in oncology have not reached FDA-approval, even though the progress has been accelerated lately. Here, two of key challenges for this class of drug (delivery and target selection) and potential strategies to overcome them are described.
– Delivery
Delivering drugs to the tissues of interest to the level high enough for strong pharmacological activity has been the biggest challenge for RNA-targeted therapeutics had. Thanks to the advances in medicinal chemistry described above, this limitation has become less critical for ASO/siRNA drugs targeting liver. Moreover, our increased understanding on how oligonucleotides are taken up by the cells on the cell surface and are processed along the endosome/lysosome pathway has greatly help to design the drugs with a better property26. However, there is still high need for improvement for the delivery of ASOs targeting tumor cells and of siRNAs targeting extrahepatic tissues/tumors.
Largely, two approaches have been explored to improve the targeting of RNA-based drugs to tumor cells. First, “passive” targeting. This strategy has no specificity for tumor types, but rather enhances the passive accumulation of drugs at tumor sites via the enhanced permeability and retention (EPR) effect. This has become the choice for siRNAs formulated with a wide variety of lipid nanoparticles to improve their distributions to the tumor cells. However, only a very small percentage of drug (0.7%) was delivered to the solid tumors while most of drugs were still trapped in the liver and kidney27. To overcome this limitation, more sophisticated delivery systems (e.g smart nanocarriers) based on the differences in pH, enzymes, and redox between tumors and normal tissues have been developed28. Even though numerous publications showing the potential of these approaches in preclinical models, it remains to be confirmed if the results can be reproducible in clinics.
The other approach is “active” targeting, which depends on the expression of cell surface proteins highly or uniquely expressed in certain tumor types. By conjugating a drug with an antibody or ligand for that particular receptor, the delivery efficiency to tumor cells can be greatly improved with reduced potential toxicity in normal tissues. This “active targeting” has been the area of active research in other drug platforms (e.g. anti-HER2-antibody-drug conjugate (ADC) for breast cancer)11, but RNA-targeted therapeutics can also benefit a lot from this approach, as demonstrated by the success in GalNAc-conjugated drugs to target hepatic genes. We can also take advantage of those successes to target tumor cells. For example, targeting hepatocellular carcinoma (HCC) with GalNAc-conjugation or GLP-1R-high tumors such as insulinoma or tumors of neuroendocrine origin with GLP-1 conjugation can be an effective way of delivering drugs to those tumor types. Assuming that not all receptors would meet the criteria (e.g. abundance, efficiency of endocytosis, and recycling time and etc.) for being sensitive to these types of conjugation approach, it will be important to identify such receptor-ligand/antibody systems bioinformatically/biochemically and eventually validate them in appropriate cell culture and animal models. The success of this approach will also be highly dependent on the medicinal chemistry, since clever modifications such as in linkers will be critical for efficient delivery to tumor cells.
– Target selection
Thus far, the majority of targets selected for the development of RNA-targeted therapeutics in oncology have been tumor cell-centric. The results that depletion of targets of interest in tumor cells leads to strong antiproliferation or induction of apoptosis in preclinical studies conducted mostly in xenograft models are typically used as key supporting data for the justification of clinical development of those targets. However, in many cases, such robust preclinical efficacy is not translated into a clinical success even though this is also the case for other drug platforms. How can we explain this discrepancy in RNA-targeted therapeutics in oncology? Given that achieving robust target depletion in tumors of patients cannot always be achievable with this technology, a target which requires near complete downregulation for strong efficacy might not be an ideal one for this technology. In this regard, identifying a target tumor cells are highly “addicted to” for their survival (it can be either an oncogene or a non-oncogene) will be crucial. For example, IRF4 is a lineage survival factor for multiple myeloma (MM), where even ~ 50% depletion of IRF4 was sufficient to lead to MM cell killing29.
Another thing to be considered in target selection is that tumor is not an isolated entity and there is constant communication between tumor cells and tumor-associated stromal cells in so-called “tumor ecosystem”. However, the importance of this cross-talk between them in promoting tumor growth is often underestimated at preclinical stages, as xenograft models in immune-compromised animals are mostly the choice for target validation. Since the amount of accumulated drugs appears to be significantly greater in tumor-associated stromal cells (therefore, likely resulting in higher activity) compared to tumor cells when systemically administered23, a target which promotes tumor growth in both tumor and tumor-associated stromal cells will be a better choice than tumor cell-centric targets in order to achieve maximal therapeutic efficacy. The relative contribution of a target from tumor cells vs stromal cells to overall tumor growth can also be easily evaluated preclinically using species-specific or cross-reactive ASOs/siRNAs. In this regard, STAT3 is a truly attractive ASO target for its tumor-promoting functions in both compartments. Alternatively, identifying and inhibiting critical target(s) important for tumor growth, but that functions primarily in tumor-associated stromal cells (e.g. immune cells, tumor-associated endothelial cells, and cancer-associated fibroblasts) can be a new strategy for taking advantage of ASO’s greater distribution to those cell types over tumor cells.
Furthermore, even though it has not been covered in this review, ASOs can also increase gene expression by sterically blocking inhibitory motif(s) on the upstream open reading frame (uORF) or preventing nonsense mediated decay (NMD)30, 31. In this regard, ASOs can be an effective approach for upregulating tumor suppressor genes whose expressions are often reduced in tumors. Finally, one of advantages siRNAs have over ASOs is their specific activity for each isoform. When a gene produces multiple isoforms with opposite functions (e.g Bcl-XS for pro-apoptotic vs Bcl-XL for anti-apoptotic), downregulating the pro-tumorigenic isoform selectively with siRNA can lead to greater antitumor activity32. Alternatively, ASOs can increase the production of pro-apoptotic isoform by altering the ratio between the two isoforms through RNase H1-independent mechanism (i.e. modulation of splicing).
6. Conclusions
Oligonucleotides-based RNA-targeting drugs have enormous potential to become a highly efficacious treatment option for the treatment of a variety of cancer types. Even though the promise has not been fully materialized yet, the emerging evidence of clinical activity as a result of advances in medicinal chemistry and our increased understanding on their delivery to tumor cells is encouraging. Therefore, it won’t be a surprise if we see their clinical success in the near future.
7. References
1. Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A 1978;75:285-8.
2. Lander ES. Initial impact of the sequencing of the human genome. Nature 2011;470:187-97.
3. Ottesen EW. ISS-N1 makes the First FDA-approved Drug for Spinal Muscular Atrophy. Transl Neurosci 2017;8:1-6.
4. MacLeod AR, Crooke ST. RNA Therapeutics in Oncology: Advances, Challenges, and Future Directions. J Clin Pharmacol 2017;57 Suppl 10:S43-S59.
5. Crooke ST, Witztum JL, Bennett CF, et al. RNA-Targeted Therapeutics. Cell Metab 2018;27:714-739.
6. Sazani P, Kole R. Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J Clin Invest 2003;112:481-6.
7. Holen T, Amarzguioui M, Babaie E, et al. Similar behaviour of single-strand and double-strand siRNAs suggests they act through a common RNAi pathway. Nucleic
Acids Res 2003;31:2401-7.
8. Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol 2017;35:222-229.
9. Geary RS, Norris D, Yu R, et al. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 2015.
10. Hong DS, Kurzrock R, Oh Y, et al. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in
patients with advanced cancer. Clin Cancer Res 2011;17:6582-91.
11. Wong DJ, Hurvitz SA. Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates. Ann Transl Med 2014;2:122.
12. Prakash TP, Graham MJ, Yu J, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency
10-fold in mice. Nucleic Acids Res 2014;42:8796-807.
13. Crooke ST, Baker BF, Xia S, et al. Integrated Assessment of the Clinical Performance of GalNAc3-Conjugated 2′-O-Methoxyethyl Chimeric Antisense
Oligonucleotides: I. Human Volunteer Experience. Nucleic Acid Ther 2019;29:16-32.
14. Nair JK, Willoughby JL, Chan A, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene
silencing. J Am Chem Soc 2014;136:16958-61.
15. Ammala C, Drury WJ, 3rd, Knerr L, et al. Targeted delivery of antisense oligonucleotides to pancreatic beta-cells. Sci Adv 2018;4:eaat3386.
16. Zimmermann TS, Karsten V, Chan A, et al. Clinical Proof of Concept for a Novel Hepatocyte-Targeting GalNAc-siRNA Conjugate. Mol Ther 2017;25:71-78.
17. Talbot DC, Ranson M, Davies J, et al. Tumor survivin is downregulated by the antisense oligonucleotide LY2181308: a proof-of-concept, first-in-human dose
study. Clin Cancer Res 2010;16:6150-8.
18. Bianchini D, Omlin A, Pezaro C, et al. First-in-human Phase I study of EZN-4176, a locked nucleic acid antisense oligonucleotide to exon 4 of the androgen
receptor mRNA in patients with castration-resistant prostate cancer. Br J Cancer 2013;109:2579-86.
19. Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver
involvement. Cancer Discov 2013;3:406-17.
20. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007;7:41-51.
21. Gide TN, Wilmott JS, Scolyer RA, et al. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin Cancer Res 2018;24:1260-
1270.
22. Hong D, Kurzrock R, Kim Y, et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma
and lung cancer. Sci Transl Med 2015;7:314ra185.
23. E.E..W. Cohen KJH, D.S. Hong, R. Mesia, I. Brana, P. Perez Segura, T. Wise-Draper, M.L. Scott, P.D. Mitchell, G.M. Mugundu0, P. McCoon, C.E. Cook, M. Mehta,
U. Keilholz. Phase 1b/2 Data of Durvalumab Plus Danvatirsen Presented at European Society for Medical Oncology. Annals of Oncology 2018;29 (suppl_8):372-379.
24. El Dika I, Lim HY, Yong WP, et al. An Open-Label, Multicenter, Phase I, Dose Escalation Study with Phase II Expansion Cohort to Determine the Safety,
Pharmacokinetics, and Preliminary Antitumor Activity of Intravenous TKM-080301 in Subjects with Advanced Hepatocellular Carcinoma. Oncologist 2018.
25. Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients.
Oncotarget 2015;6:24560-70.
26. Liang XH, Sun H, Shen W, et al. Identification and characterization of intracellular proteins that bind oligonucleotides with phosphorothioate linkages.
Nucleic Acids Res 2015;43:2927-45.
27. Rosenblum D, Joshi N, Tao W, et al. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun 2018;9:1410.
28. Zhang P, An K, Duan X, et al. Recent advances in siRNA delivery for cancer therapy using smart nanocarriers. Drug Discov Today 2018;23:900-911.
29. Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature 2008;454:226-31.
30. Liang XH, Shen W, Sun H, et al. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat
Biotechnol 2016;34:875-80.
31. Huang L, Low A, Damle SS, et al. Antisense suppression of the nonsense mediated decay factor Upf3b as a potential treatment for diseases caused by nonsense
mutations. Genome Biol 2018;19:4.
32. Trisciuoglio D, Tupone MG, Desideri M, et al. BCL-XL overexpression promotes tumor progression-associated properties. Cell Death Dis 2017;8:3216.
☞ 자세한 내용은 내용바로가기를 이용하시기 바랍니다.
지식
동향

