기술동향
PROTACs: Reinvention of an old workhorse-the small molecule drug
- 등록일2019-12-11
- 조회수5889
- 분류기술동향
PROTACs: Reinvention of an old workhorse-the small molecule drug
Department of Pharmaceutical Sciences, University of Kentucky
Kyung Bo Kim, Ph.D.
Background
The successful completion of human genome project and proteome information has revealed a myriad of proteins that may be associated with many of the deadliest diseases, potentially offering an enormous opportunity to intervene pathological processes. However, most of these proteins are deemed as “undruggable” due to the lack of defined binding pockets that can be functionally blocked by existing drug modalities. Currently, conventional small-molecule and antibody drugs can access only about 20% of these proteins.1 As a result, expanding the landscape of druggable protein targets further into previously non-druggable disease-
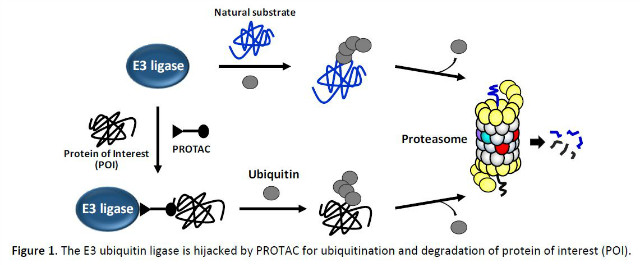
promoting proteins has long been a major challenge for the pharmaceutical industry.
Developed and crafted over the past decades, a revolutionary small-molecule family called “PROTACs” could be an answer to this conundrum for pharmaceutical industry. The PROTACs (Proteolysis Targeting Chimera) work by cleverly hijacking a cell’s natural quality-control system and using it for destroying proteins of interest (Figure 1).2 Because PROTACs (“baits”) simply need binding rather than inhibiting proteins of interest (“preys”) to work, they could conceivably target non-enzymes or non-receptors: proteins that are lacking functionally relevant ligand-binding pockets and thus are largely out of reach for drug development. Another important feature of PROTACs is their sub-stoichiometric catalytic nature,3 thereby making them distinctively different from conventional drugs that are used in great stoichiometric excess of their targets to be effective. New modalities such as RNA-based silencing and gene editing can also turn-off the activity of previously unreachable protein drug targets. However, they are routinely accompanied with some inherent issues, such as delivery, storage and formulation, which make the drug development process excruciatingly long and often financially untenable. In comparison, PROTACs being small molecules in essence potentially face fewer hurdles to reaching the clinic door and also could be mass-produced, stored long in pill form. With a variety of potential protein targets for intervention, the PROTAC platform could be applied to various disease states, from eliminating hormone-dependent tumors to clearing clusters of disease-causing proteins from the brains of patients with neurological disorders including Alzheimer’s disease. The PROTAC platform is rapidly gaining momentum now that the pharmaceutical industry, attracted to the potential to intervene ~80% of the proteome untapped for drug discovery, is pouring billions into the field.
History: The creation of PROTACs
The idea to target undruggable proteins using cell’s own protein degradation machinery (UPS, Ubiquitin-Protein System) was initially brewed over chatting between Prof. Craig Crews of Yale University and Prof. Ray Deshaies of Caltech (now senior vice president of Amgen) during a Burrow Welcome Fund sponsored research retreat in 1998 at a resort on Semiahmoo Bay in northwest Washington. Started with chatting over poster sessions and continued the conversation over beers all weekend, the idea of linking E3 ligase (β-TRCP, an F-box protein) to UPS using chimeric molecules for ubiquitination and degradation of targeted proteins was soon realized by a PNAS publication by Crews’ and Deshaies’ groups.4 Because first-generation PROTACs were composed of a large phosphopeptide ligand for an E3 ubiquitin ligase (β-TRCP) on one end and a small molecule ligand that binds to proteins of interest on the other end (Figure 2a), they were only deliverable vie microneedle and also had weak activity in human cells. As a result, the concept of PROTACs was for years regarded as an academic exercise. Unfazed by initial skepticism, Crews and colleagues continuously worked to improve the PROTAC platform, eventually expanding the pool of E3 ligase to VHL (Von-Hippel Lindau) protein which recruits HIF-1α under normal oxygen concentration condition to induce ubiquitination and degradation to shut down cellular hypoxic responses.3 The replacement of β-TRCP ligand with a shorter non-phosphopeptide fragment derived from HIF-1α improved pharmaceutical properties of PROTACs to a certain degree (Figure 2b), but there remained much room for improvement.
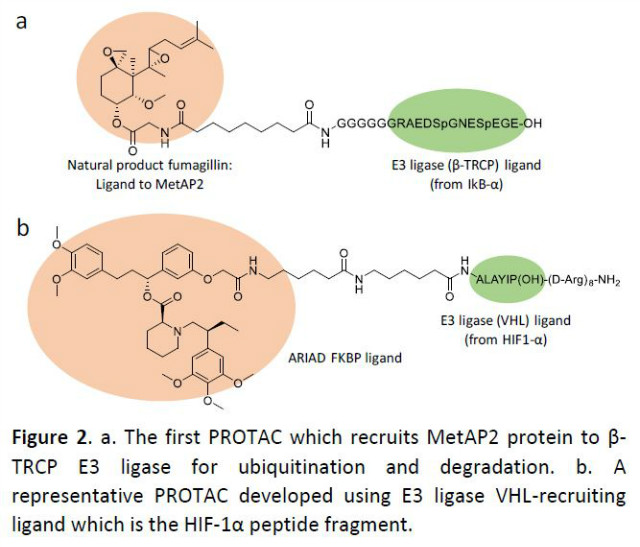
Realizing the potential of the PROTAC platform, in 2013, Crews founded Arvinas, Inc. in New Haven, CT, USA. While the field were still confronted with some doubts, the idea to transform the PROATC concept into marketed treatment finally got a big boost: revelation of PROTAC-like mode of action for some of world’s best-selling drugs. A series of studies performed in 2010 and 2015 revealed that the multiple myeloma drugs thalidomide and lenalidomide (Revlimid®, an amino analog of thalidomide that’s been a multi-billion dollar drug for Celgene) bind to the cereblon (CRBN) E3 ligase and induce ubiquitination and degradation of transcription factors (Ikaros and Aiolos) that are critical for myeloma cell growth.5-6 The thalidomide-based protein-degrading platform was further verified by additional studies that modification of thalidomide alters the binding partners of CRBN, inducing degradation of casein kinase 1α (CK1α) and a translation termination factor GSP1 that are otherwise not targeted by CRBN.7-8 All of sudden, this has made the protein degrading platform one of the hottest new technologies for developing new drugs, opening a flood gate for new biotech businesses.
...................(계속)
☞ 자세한 내용은 내용바로가기 또는 첨부파일을 이용하시기 바랍니다.
지식
동향
- 기술동향 표적 단백질 분해 기술(TPD, Targeted protein degradation) 및 그 파생기술 연구 동향 2022-12-08
- 기술동향 SMC proteins 2017: Chromosomal organizers from bacteria to human 참관 후기 2017-09-19
- 기술동향 System biology 동향: Protein-protein interaction network 구축현황과 활용 2017-03-29
- 기술동향 단백질 칩의 응용과 활용전망 2014-01-08
- 기술동향 Non-coding antisense RNA can be used to stimulate protein production 2012-10-23

